How It Started and How It’s Going : A Scientific History of the Origins of Life Research, Part 1
From "Warm Little Pond" to RNA-World
“It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present.— But if (& oh what a big if) we could conceive in some warm little pond with all sorts of ammonia & phosphoric salts …..”
Charles Darwin, Letter to J. D. Hooker, 1871.
1. “..if (& oh what a big if)”
One of the weirdest facts of life science is that there is no unanimous definition of ‘Life’.
The difference between living and non-living things appears to be very simple. It is one of the first categorical demarcations we become aware of as kids. We think we know life when we see it. Then we learn that the trees in our garden are also living beings like our pet dog, Puffy, and we experience a conceptual expansion. Unknowingly, we had affirmed Aristotle’s scala naturae: his “ladder of life” placed rocks at the bottom, plants in the middle, and humans at the top. Among scientists, the definition of Life is a matter of high consensus but not complete unanimity. Are viruses alive? What about prions? Artificial intelligence has created new tensions.
These disagreements seem relatively amicable when compared to the debate over where life comes from. Well-informed people, both inside and outside the scientific community, see the field of origins-of-life research as trying to invoke a miracle.
But in dozens of laboratories spread across the world, experimentalists are testing equations, measuring how much energy pre-life reactions require, counting how many bits of sequence survive the next replication cycle, and pondering how persistence wrestles with entropy, all in an effort to advance a theory of how life came to exist in the universe and specifically on planet Earth.
There will be no equivalent of the egg-hatching scene from Jurassic Park under a microscope. There will be no minuscule prebiotic Frankenstein’s monster being animated with a jolt of electric discharge from an electron lamp. And that’s fine. The more interesting question is how far we can get before mystery takes the wheel. The answer, after a century and a half of work, is: farther than most people think.
2. In the Beginning..
..was, like with so much of modern biology, Darwin. Darwin proposed “warm little ponds” as a site for abiogenesis (the emergence of life from non-life) in 1871. He hinted at the unilluminated path between chemistry and biology, and the view he was espousing would come to be known as abiogenesis by chemical evolution. His proposal was in a private correspondence and not in a scientific publication. But it was Darwin’s private correspondence. So “warm little ponds” would resurface as a touchstone when scientists began to think seriously about abiogenesis by chemical evolution.
The other contemporary competing doctrine was of spontaneous generation: “maggots from meat.” The doctrine, originating in antiquity with Aristotle, claimed that fully formed life (flies, mice, microbes) could appear directly from rotting matter or broth without precursors. It was obvious: leave a flask of bone-broth out on a windowsill, and within days it teemed with life. How could anyone doubt it?
Louis Pasteur did. Yes, the same Pasteur who pioneered vaccines and pasteurization and would go on to save millions of lives. But in the 1850s, he was dismantling spontaneous generation through his ingenious experiment. He filled glass flasks with nutrient broth and heated them to kill anything already inside. His ingenuity was in the glasswork: each flask had a long, swan-necked curve, open to the air but bent in such a way that dust and airborne particles settled harmlessly in the bend, never reaching the broth. For weeks, the broths remained clear, sterile, lifeless. But when Pasteur tilted a flask so dust slipped into the liquid, microbes bloomed. The verdict was decisive: life did not arise spontaneously in broth. It arrived with microbial stowaways in the air.
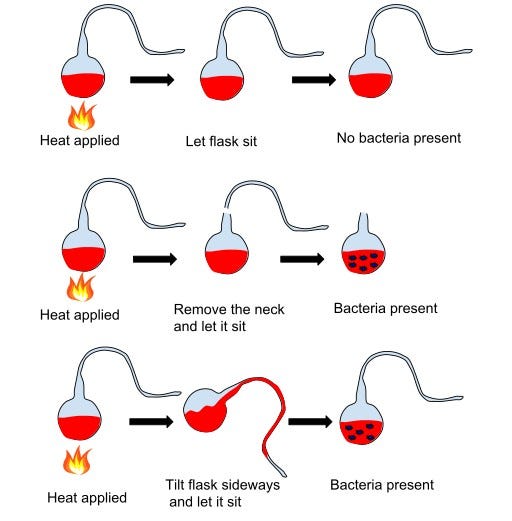
Pasteur’s demonstration demolished spontaneous generation as an informed explanation for life’s appearance. From then on, “life comes from life” became the new axiom. But this also reframed the puzzle. If no amount of bone broth would ever sprout new organisms, then the origin of life could not be an everyday occurrence. It must have been an extraordinary set of conditions, long ago, where chemistry was pushed across boundaries into biology. That’s where Darwin’s prescient observations come in. He even noted why we don’t see this tentative transition happening today: the modern world is too crowded with hungry organisms that would devour such fragile beginnings.
Pasteur had shown what could not happen in ordinary conditions; Darwin proposed what might have happened in extraordinary ones. Together they fixed the terms of the debate: not epochal miracles, not everyday broth, but something in between, something still out-of-the-ordinary: Extraordinary chemistry in extraordinary settings.
3. Extraordinary Claims
Despite the pioneering work in other areas of biology (evolution, genetics, biochemistry), by the early twentieth century, the problem of life’s origin had slipped into the background. The origin of life problem was too speculative for most scientists to risk their reputation. The subject lay dormant, waiting for someone reckless enough or imaginative enough (or both) to pick it up.
Not one but two such figures appeared in the 1920s.
In Moscow, the young biochemist Alexander Oparin proposed that the early Earth had been wrapped in a “reducing” atmosphere rich in methane, hydrogen, ammonia, and water vapor. In such a world, he argued, simple molecules could build into more complex organic compounds. Once formed, these compounds could cluster into microscopic droplets termed “coacervates” – tiny, cell-like blobs that concentrated chemicals and even carried out rudimentary metabolism.
Across the continent and beyond the channel, in Britain, the polymath JBS Haldane was sketching an almost identical picture. He imagined a planet bathed in ultraviolet light, hammered by storms, and slowly filling its oceans with a “primordial soup” of organic molecules. In this chemical broth, he suggested, the precursors of life could accumulate until a threshold was crossed and evolution could begin.
Together, these men turned Darwin’s private musing about “warm little ponds” into a framework, a kind of research program. Although none of them had any experiments to show, they changed the nature of the puzzle from philosophical to a chemical one. They gave later scientists hypotheses that could be tested, predictions that can be challenged in glassware and electric discharges.
4. Extraordinary Evidence?
Glassware and electric discharges were exactly what the young graduate student Stanley Miller employed in 1952 to test the hypothesis of whether simple gases could give rise to the molecules of life.
Working under Nobel laureate Harold Urey at the University of Chicago, Miller built a contraption that looked more like plumbing than chemistry. Glass flasks and tubes looped together in a closed circuit. At one end, he sealed a mixture of gases he believed resembled the early Earth’s atmosphere: methane, ammonia, hydrogen, and water vapor. At the other end, he installed electrodes that fired continuous sparks, simulating lightning storms. The gases circulated, condensed, and boiled again, day after day, as the artificial storm raged inside the glass.
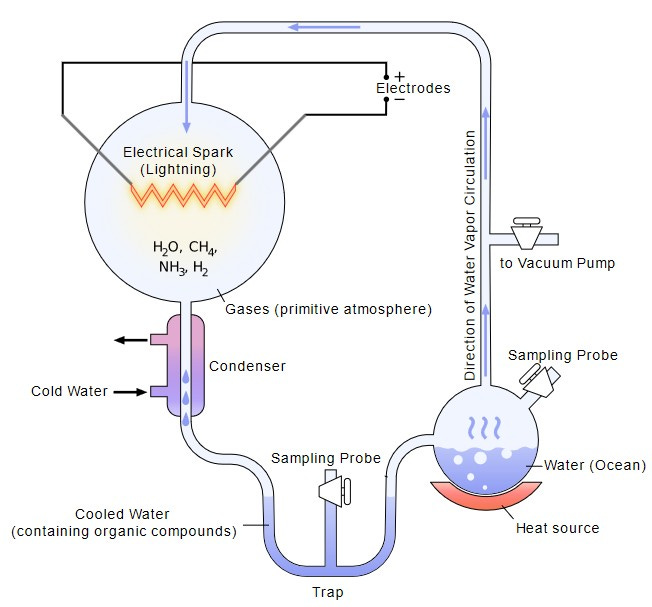
After a week, Miller opened the apparatus to check what had formed. The clear water had turned a murky reddish-brown. Chemical analysis revealed why: the experiment had produced amino acids (the building blocks of proteins) and urea (a waste product of “organic” processes inside a living being) out of nothing more than simple gases and electrical energy.
The results electrified the scientific world. Here was a direct test of the Oparin–Haldane idea, and it had delivered. While Friedrich Wöhler had synthesized the organic molecule urea back in 1828, it didn’t deal a death blow to the prevailing theory of the era. This was called “vitalism,” which held that there was something special, something “vital” about molecules that make up living beings (“organic” molecules). If this were true, a person could not make organic molecules from inorganic ones. (In a kind of argument that will be instantly recognizable to scientists everywhere, the proponents of vitalism would argue that urea didn’t count because it was an inert waste product of living processes.)
Miller's experiment went further. He had not produced a simple metabolic waste product, but amino acids – the fundamental building blocks of proteins. Any lingering philosophical arguments for a 'vital force' were rendered irrelevant by the sheer biochemical significance of creating the core components of life from simple gases in a flask.
There were caveats, of course. Later research suggested that Earth’s early atmosphere may not have been as strongly reducing as Miller assumed. And amino acids, though essential because they link together to make proteins, are only one part of the puzzle. Life also requires nucleotides (for making DNA), lipids (for making cell walls), and mechanisms of replication (for heredity).
But none of the caveats dulled the impact. The “primordial soup” had been condensed from the vapor of metaphor and caught in a lab glassware. The Miller–Urey experiment turned a new page. Abiogenesis was now shown to be experimentally tractable.
With enough imagination and the right equipment, scientists could now begin to test scenarios for life’s beginning. Encouraged by Miller’s spark, other laboratories extended the recipe. Hydrogen cyanide chemistry produced adenine, one of the nucleobases that make up DNA and RNA. Formamide yielded a variety of nucleobases as well. Simple lipids, capable of forming membranes, were shown to arise under prebiotic conditions.
Piece by piece, the building blocks of biology (amino acids, nucleotides, and lipids) proved accessible to plausible geochemistry. But building blocks alone were not enough. A pile of bricks does not a house make. For that, you need a blueprint; you need information.
5. The Information Problem
Within a decade of Miller’s experiment, one could make the molecules of life in a lab under plausibly prebiotic conditions. But molecules are not life. Life needs a way to preserve useful sequences, to copy them reliably enough that improvement can accumulate. The questions now were shifting. How do these molecules preserve information across generations? How does information survive the noise of imperfect copying?
By the late 1960s, the problem wasn’t chemistry anymore; it was information. And this was the problem that Manfred Eigen put into equations, and Sol Spiegelman tested at the bench.
Drawing on Claude Shannon’s information theory, Manfred Eigen, a German biophysicist, showed that replication has a brutal constraint: if copying is too sloppy, information dissolves into noise. He called this boundary the error threshold. Below it, hereditary information can survive across generations; above it, evolution collapses. Here was nature red in tooth and claw, even at the information-theoretic level.
To explain how early replicators could sustain themselves, Eigen proposed the idea of “quasispecies” – swarms of related sequences clustered around a “master” sequence. He even imagined hypercycles, networks of replicators that catalyze one another, as a way to stabilize complexity against parasites.

So here was the mathematics. But where was the experiment?
Enter Sol Spiegelman, an American molecular biologist with a flair for experiments that bordered on performance art. In 1965, he extracted the RNA genome of a bacteriophage (a virus that infects bacteria) and used an enzyme called Qβ replicase to copy the RNA. With each transfer to a fresh test tube containing the replicase and the chemical building blocks for replication, the RNA's only job was to get copied. Soon, the long viral genome had slimmed down to a much shorter molecule that replicated faster. Stripped of pretenses and given all the resources, biological information only seemed to care about faster replication. Selfish, indeed.
Spiegelman had conjured what the press dubbed “Spiegelman’s Monster”: an RNA parasite that shed everything non-essential in its need for speed. It was both thrilling and sobering. Here was evolution in real time, naked and molecular. But it was also a warning: without constraints, replicators collapse into parasites or gibberish. Information has to be protected from entropy if life is to get anywhere at all. (This is a thread we will pull on later in this series of essays.)
The origins-of-life research has now gone from infancy to toddlerhood, from making molecules to understanding what makes them persist. The problem had shifted from “Can we build the bricks?” to “How do the bricks remember the shape of the house?” and “How do they make themselves into a house?”
6. The Catalyst in the Code
Eigen’s equations and Spiegelman’s monster had made the stakes clear: heredity mattered, but it was fragile. And the machinery of life can now clearly be divided along the metabolism-information axis. Some molecules did the work (proteins) but can’t remember their recipes, and some molecules carried instructions (DNA and RNA) to make the molecules that did the work, but were helpless on their own. Information was information, metabolism was metabolism, and never the twain shall meet in a single molecule. Or do they?
The next question was obvious: What molecule first carried both information and function? Life as we know it seemed to run on a one-way street from information to function: DNA to store, RNA to transfer, proteins to act. Evolution needed a starter system.
In the early 1980s, a stunning picture began to emerge. Researchers Thomas Cech from Colorado and Sidney Altman from Yale independently discovered that RNA was not just a passive messenger between DNA and protein. Certain specific RNA molecules could act as an enzyme in their own right – a function previously thought to be exclusive to proteins. Cech found a piece of RNA in a microorganism that could cut and splice itself without any help from proteins. Altman found that the RNA component of a bacterial enzyme was the active, catalytic part. So RNA was revealed to be a class of molecules that could both store information and perform a function, and such RNA was named a ‘ribozyme’ (a portmanteau of ribonucleic acid + enzyme). This was the bombshell: a single type of molecule could, in principle, run the whole show.
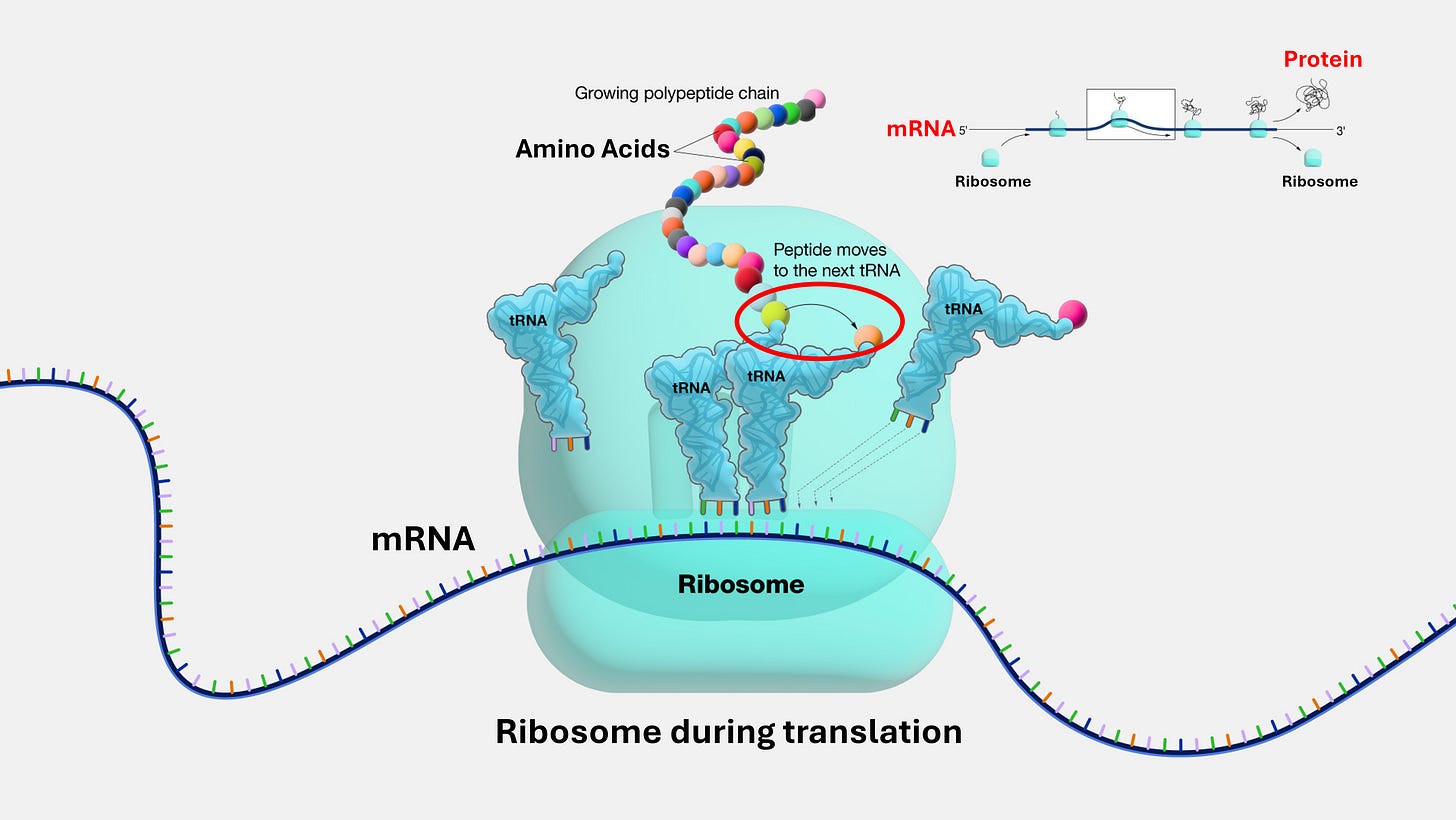
The most profound evidence for this ancient world of RNA exists today, operating at the very heart of all life. It’s found in the ribosome, the assembly line inside every cell's protein-building factory. The ribosome is a complex biomolecular structure that reads genetic instructions from a messenger RNA (mRNA) blueprint and links amino acids together to create a protein. For decades, it was assumed that the proteins in the ribosome did this crucial work. But they don't. The catalytic engine of the ribosome, the part that actually forges the bonds between amino acids, is pure RNA. The ribosome is the ultimate ribozyme, a molecular fossil proving that information and metabolism both can be, and are still, run by RNA.
The idea caught fire in 1986 when Walter Gilbert, a Harvard molecular biologist, gave it a name: the RNA World. The field now pivoted from chemistry (Miller-Urey) and information (Eigen-Spiegelman) to catalysis, with RNA at the center.
7. One Ribozyme to Rule Them All
Origins-of-life research now had a new focal point. The task was no longer abstract: if RNA could store information and catalyze chemistry, perhaps early life was built on RNA alone. An RNA World could solve the chicken-and-egg problem of which came first. Gilbert argued that before DNA and proteins carved up the labor of life, RNA might have been the one molecule to do both jobs. RNA could store information and catalyze reactions at the dawn of life.
The challenge now was practical and the questions experimentally testable. Could RNA catalyze its own replication? Could it sustain itself (and hence evolution) without proteins?
These questions launched whole new experimental programs. In the 1980s and 1990s, laboratories began evolving ribozymes in test tubes, pushing RNA to see how far it could go. They selected RNA molecules that could cut and ligate other RNAs, or even join nucleotides into short chains. Progress was steady but incremental. None of these ribozymes could yet string together long enough chains to copy themselves.
Then, in 2009, Gerald Joyce and Tracey Lincoln of Scripps Research Institute took a dramatic step forward. They engineered a pair of ribozymes that could catalyze each other’s synthesis from smaller RNA fragments–reminiscent of Eigen’s hypercycles. This wasn’t “self”-replication in the strictest sense, as it required two partners in a molecular “cross-replication” dance. But it proved that RNA molecules could, under the right conditions, sustain exponential amplification. For the first time, RNA evolution could be watched in real time, away from the meddling arms of protein enzymes.
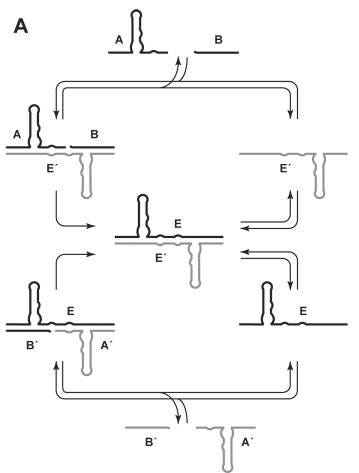
Still, limitations remained. The ribozymes worked only with carefully designed fragments, not arbitrary sequences. Their speed and fidelity were too low to climb over Eigen’s error threshold. But the principle was there: RNA could be both information and machine, and evolution could run on that alone. The RNA World had teeth.
But one obstacle to acceptance of the RNA World refused to budge: RNA itself. Too bulky to appear pre-assembled, too unstable to assemble cleanly from bases, sugars, and phosphates, it seemed beyond the reach of plausible prebiotic chemistry. The field needed a path forward, and it came in the form of an imaginative reaction pathway that showed where the building blocks could have come from in the first place. That is where Part Two will begin: with a chemist in Cambridge, John Sutherland, and a fresh way to engineer life’s first molecules, before following the parallel threads of compartmentalization, panspermia, chirality, energy, and LUCA: Last Universal Common Ancestor.
With gratitude to Mike Riggs for his thoughtful editing, and to Sarahi Enriquez, Alexander Kustov, Ariel Patton, Ibis Slade, Deric Tilson, and Elizabeth Van Nostrand for the valuable feedback during the writing process. Your ideas and critiques made this piece better.



