How It Started and How It’s Going: A Scientific History of the Origins of Life Research, Part 2
The Crossing of Thresholds.
The mysterious questions of origins (of Life, Universe, Everything) are a precursor to myth and philosophy. Humans have pondered the origin of life for millennia. It was only a matter of time before science, another uniquely human endeavor, would be employed to decipher this most mystical of mysteries. And so it began in the mid-19th century. This series of essays tells the scientific history of the origins-of-life research. Here is Part 1, which I have also summarized below.
The story so far:
In the previous part, the narrative began with the first naturalistic speculation on the question of life’s origin: Darwin’s “warm little pond” hypothesis. Life, Darwin proposed, could arise from a mix of simple chemicals in favorable conditions, setting science apart from supernatural speculations. Then Pasteur’s experiments swiftly disposed of the idea of spontaneous generation, proving that life arises only from existing life under natural conditions. So all life originates from extant life. But the first life, by definition, must have started from non-life.
The conceptual torch then passed to Oparin and Haldane, who proposed that Earth’s early, reducing atmosphere allowed complex organic molecules to form through gradual chemical evolution. This theoretical proposal was experimentally supported by Miller and Urey, who generated amino acids by shocking primitive gases with electrical sparks. This inspired a series of experiments that led to the generation of biologically relevant molecules under plausibly prebiotic conditions.
Moving beyond mere molecule generation, the focus shifted to information: Manfred Eigen calculated error thresholds for heredity, showing that only sufficiently high-fidelity replication could support the maintenance and evolution of genetic information. Spiegelman’s experiments exposed the dark side of fast-replicating RNA, as parasitic sequences quickly dominated in vitro, underscoring the apparent antagonism of replication and metabolism. The next leap emerged with Cech and Altman’s discovery that RNA can catalyze reactions, revealing a plausible prebiotic route for self-sustaining chemistry.
RNA molecules with catalytic functions, named ribozymes, seemed to be an exciting candidate for a plausible proto-molecule at a time when information storage and metabolism were not yet distinct functions. These ribozymes (coined from ‘RiboNucleic Acid + Enzyme’) were capable of doing both. Walter Gilbert branded this paradigm the “RNA-World,” capturing scientific and public imagination.
Throughout the 1980s–2000s, laboratories succeeded in evolving increasingly sophisticated ribozymes. This crescendoed in the Joyce–Lincoln experiment, which demonstrated cross-replicating RNA molecules that amplified themselves without the need for proteins, approaching a minimalist model for early life.
This series of breakthroughs can be interpreted as a collection of threshold crossings: from non-life to chemistry, chemistry to information, information to replication, and replication to compartmentalization. However, these were not a linear progression of events. Origins-of-life research increasingly shows that these thresholds do not represent neat, sequential steps along a straight path. Instead, they form an interwoven network of intertwined processes. These essays are aimed at unpacking how these intertwined transitions gradually transformed a world of chemistry into one of biology
When we left the story in the previous part, RNA had taken center stage. It could store information, catalyze reactions, and (at least in principle) evolve without proteins. Yet it remained an implausible hero. RNA was too large, too fragile, and too difficult to assemble from the simple molecules available on the early Earth. The theory lacked chemistry.
8. Brave New RNA-World
RNA is a long chain, or polymer, made of a backbone of alternating sugar and phosphate molecules. Attached to each sugar is one of four nucleobases, which carry the genetic instructions. The sugar is called ‘ribose’, and the phosphates give the entire molecule its acidic properties, hence the name RiboNucleic Acid. In other words, RNA is too cumbersome a molecule to spontaneously appear fully formed on prebiotic Earth.
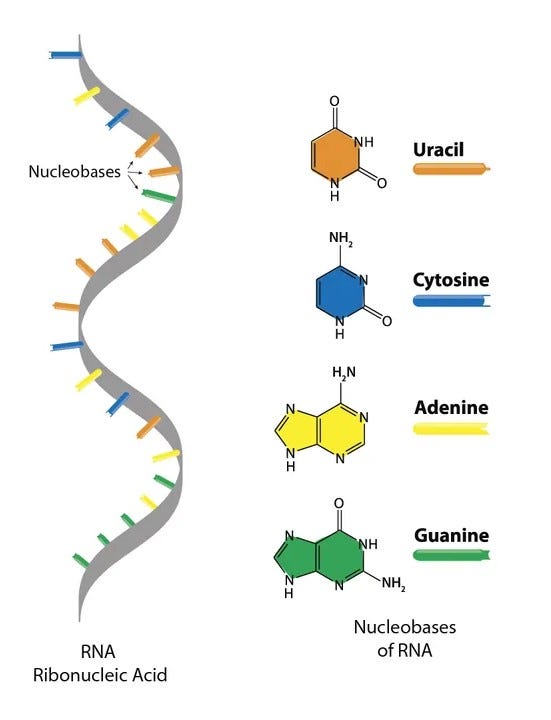
So, for all the excitement around ribozymes, one hard question remained hanging in the air: even if RNA could, in principle, copy itself, where would the first RNA molecules have come from?
For decades, chemists tried to assemble nucleotides by stitching together the three obvious parts (base, sugar, and phosphate) without success. Chemists pursued this classical approach for so long because it mirrored the intuitive assumption that nucleotides must be assembled from their parts. After all, this is how complex biomolecules form stepwise in biology today. It was natural to expect that prebiotic nucleotide formation followed similar logic. So, early synthetic organic chemistry often relied on stepwise, linear reactions combining defined fragments under controlled conditions.1
But the reactions stubbornly refused to cooperate under realistic prebiotic conditions.
To understate the challenge: under prebiotic conditions, the sugar ribose itself was unstable, the bond to the base rarely formed cleanly, and phosphate chemistry was inelegant. To elaborate: You start with an unstable ingredient that barely exists (ribose), try to force a bond that doesn’t want to form in water (glycosidic bond), and finish with a messy, non-specific reaction (phosphorylation).2
This impasse left the RNA-World hypothesis vulnerable. It was elegant in theory but stuck on a practical absurdity: how could nature have built such a complex molecule without help?
The stalemate lasted until 2009, when John Sutherland and his colleagues in Cambridge reframed the problem. Their approach was reminiscent of the heady decade after Urey-Miller’s experiment, but with the brilliant flash of insight of applying ‘systems chemistry’ instead of linear synthesis. Systems chemistry approach is about exploring the simultaneous, interconnected reactions occurring in complex mixtures rather than isolated, stepwise assembly.
Instead of attempting to build biomolecules piece by piece, prebiotic systems chemistry relies on networks of reactions that interact, feed back, and co-evolve in ways that can lead to emergent properties, such as self-organization and autocatalysis. This approach mimics the messy, dynamic chemical environments likely present on the prebiotic Earth.
So instead of trying to glue bases onto pre-made sugars, Sutherland and his team showed that nucleotides could be built up step by step from simpler precursors (hydrogen cyanide, cyanamide, phosphate) that were plausible on the early Earth.3 Crucially, the reactions didn’t require contrived conditions. They worked under UV light in simple solutions.
The significance was not just that RNA’s parts could form – we already had evidence for each individually. It was that they could form convergently alongside amino acids and lipid precursors from the same chemical feedstocks. Suddenly, the “RNA is too big to make spontaneously” objection looked less damning. While the problem of how to ‘activate’ these monomers and string them into long chains remained, the source of the building blocks themselves now seemed far less mysterious. Prebiotic Earth appeared to have more chemistry up its sleeve than scientists had once imagined.
But chemical plausibility is not the only precondition for prebiotic chemistry to happen; physical proximity of the reacting species is paramount too. It is not enough to have ingredients in their separate boxes on different aisles in the supermarket. Cooking up life, like cooking any recipe, requires all the ingredients to be in the same pot. This means activated nucleotides in one place, amino acids and lipids nearby, all jostling in the same environment.
This requirement for proximity raised a new question: what natural settings could gather, concentrate, and hold onto these fragile molecules long enough for the next steps to happen?
9. Holding It All Together
Molecules left to drift freely would simply dilute into the ocean, their concentrations dropping below useful levels. A replicator that manages to copy itself in open water is still doomed if it cannot hold onto its products. Life demands not only information and chemistry but also a way to keep everything in one place.
The simplest boundaries turned out to be the easiest to imagine: fatty acid vesicles. Fatty acids, a simple type of lipid, are long hydrocarbon chains with a carboxyl group attached to one end. Carboxyl groups are ‘polar’, i.e., they love water. Hydrocarbon chains, being non-polar or ‘oily’, do not. When you have enough fatty acids in water, they will form a bilayer, like a sandwich, with the water-hating hydrocarbon chains facing inside, away from water. This is a lipid bilayer.
In the 1990s, researchers like David Deamer showed that simple lipids, which can form spontaneously from prebiotic-like chemistry, self-assemble into bubbles.4 These vesicles enclosed water on the inside, separated from the outside by a delicate film. They were fragile, but surprisingly lifelike. Feed them more fatty acids and they grew. Agitate them and they divided. Here were primitive cells without a single protein.
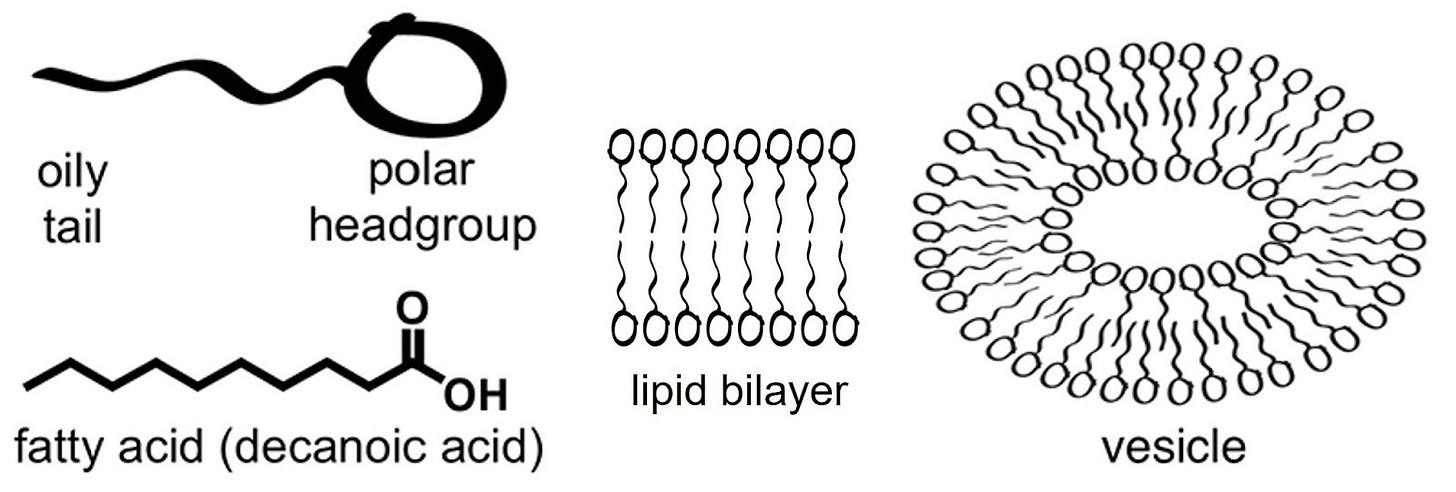
But membranes created new tensions. RNA replication requires magnesium ions, and magnesium tends to rip fatty acid vesicles apart. For years, this seemed like an incompatibility. What is the point of having non-replicating RNA imprisoned in a shell? What is the point of having RNA drifting free in the oceans if they can’t come together to self-replicate? The chemistry of heredity and the physics of containment worked at cross-purposes.
Then Jack Szostak’s group found a workaround: citrate, a simple organic acid, could ‘chelate’ (science-speak for sequester) the magnesium, protecting membranes while still allowing RNA to copy itself.5 Suddenly, the two branches of origins research, replication and compartments, were no longer enemies. They could coexist in the same protocell.
In parallel, another tradition revived an older idea: coacervates. First imagined by Oparin in the 1920s, these droplets form when polymers such as RNA and peptides spontaneously separate from water into dense, cell-like blobs – a phenomenon called phase separation. Modern experiments show that coacervates can concentrate molecules, accelerate reactions, and even maintain distinct internal environments. Under the microscope, they twitch, merge, and bud – yes, it looks uncannily cellular.6
Whether vesicles or coacervates, the point is the same. Compartmentalization was a critical threshold to cross on the way from chemistry to biology. Compartments enabled the trapping of reactants, keeping replicators together long enough for evolution to work, and allowing selection to act not just on individual molecules but on whole protocells. Once compartments enter the scene, chemistry is no longer spread thin across an ocean. It is bottled, focused, and persistent.
With compartments secured, the picture of early life sharpened. But the problem was still incomplete. Containment and replication are powerless without a steady fuel supply. Life does not just persist: it persists against entropy. The next question was how protocells powered their chemistry. Where did the energy come from? And how was it captured?
10. The Non-Negotiability of Energy
Energy is the non-negotiable currency of life. Information and compartments are crucial, but they are powerless without energy. Replication and heredity may explain continuity, but without a steady supply of energy to push against entropy, protocells would collapse. Life, at its core, is not a static arrangement of molecules but a system that keeps itself away from equilibrium. Equilibrium is death; death, equilibrium.
One early vision came from the German chemist Günter Wächtershäuser, who in the 1980s championed the iron–sulfur world. He pictured primitive metabolisms starting on mineral surfaces, with sulfides and metals like pyrite driving reactions. In this scheme, life began not with nucleic acids but with geochemistry: self-organizing reaction networks that gradually grew more elaborate until they folded in genetics. It was a “metabolism-first” theory: life as chemistry on the move before it was ever life as information.7
Then came the vents.
In the late 20th century, oceanographers discovered alkaline hydrothermal fields. The most famous of these, the Lost City Hydrothermal Field, was identified in the mid-Atlantic in December of 2000.8 Unlike the black smokers that belch scalding water, Lost City’s towers vent warm, alkaline fluids into the acidic ocean. The mixing creates a natural proton gradient across thin mineral walls – essentially, a geological battery.

Every modern cell, from bacteria to humans, runs on chemiosmosis: proton gradients across membranes power ATP synthase, the rotary machine that makes life’s universal energy currency. Biochemists Bill Martin and Nick Lane argued that this similarity is fundamental. The universality of proton gradients suggested inheritance. In this view, hydrothermal vents and the first protocells exist on a continuum. Cells didn’t invent gradients – they co-opted them.9
This is a powerful argument, though not the only one. Some researchers argue this is a classic case of convergent evolution: proton gradients might simply be the best (or the only) robust physical solution for powering a cell, meaning all life that survived would have had to adopt it independently, not necessarily inherit it from a vent.10
Still, the vent model reframed the problem. Instead of protocells somehow “discovering” energy management after the fact, they may have been born in energy-rich settings. Gradients came first; membranes and heredity learned to capture and control them later. The logic was stark: no gradients, no persistence.
Energy, once a side note in origins debates, became central. Replication and compartments provide the form of life. Gradients supply the force that keeps it running.
11. Stardust in the Recipe
While geochemists drilled into vents and biochemists debated membranes and gradients, another kind of evidence arrived from the sky.
In 1969, a fireball broke apart over Australia and scattered fragments across the countryside near Murchison. The Murchison meteorite turned out to be a chemical treasure chest: more than seventy amino acids, including many not used in life today, plus nucleobase precursors, simple sugars, and a menagerie of other organics.11
Decades later, the European Space Agency’s Rosetta mission visited comet 67P/Churyumov–Gerasimenko. We filmed the entire thing12. Rosetta’s Philae lander detected glycine and phosphorus on the comet’s surface. Other analyses of interstellar dust have revealed similar complexity.
The cosmos, it seemed, was littered with prebiotic chemistry.

The discoveries were thrilling but also misleading. They inspired a revival of panspermia (the idea that life itself may have come from space). But meteorites and comets do not solve the origin problem. They only move it elsewhere. If life rode here on a comet, where did it arise before hitching that ride? And if it arose out there, then it must have crossed the same chemical thresholds we are trying to understand here.
The sober conclusion is that space enriches the pantry, but doesn’t write us the recipe. Extraterrestrial organics could have jump-started Earth’s chemistry by delivering critical feedstocks in bulk. But the step from molecule to metabolism, from chemistry to heredity, still had to happen on this planet. The rain of stardust stocked the shelves – but the cooking took place in Earth’s own kitchens.
12. The Chirality Riddle
One more puzzle lurks inside those meteorite organics. When scientists analyzed the amino acids in the Murchison meteorite, they found something remarkable: the mix was not perfectly even. A small but measurable excess of left-handed amino acids appeared – precisely the same handedness that life on Earth uses.
This mattered because life’s chemistry is chiral. Its building blocks come in two mirror-image forms, like left and right hands. In the laboratory, prebiotic reactions almost always make racemic mixtures – equal parts left and right. But biology chose just one hand: L-amino acids for proteins, D-sugars for nucleic acids.
Why? And how was the choice enforced?
Meteorites hinted at an answer: perhaps the cosmos delivered Earth not only molecules, but also a bias. That bias needn’t be large. Just enough for amplification mechanisms on Earth to push it to exclusivity. Chemists have demonstrated how this could happen.
The Soai reaction, discovered in the 1990s, showed that certain asymmetric autocatalytic reactions can amplify a tiny excess into nearly pure homochirality. The process called Viedma ripening demonstrated another route: crystals of chiral molecules can dissolve, reform, and grind against one another until only one hand remains.13
Neither mechanism is “the answer,” but both prove the principle: symmetry-breaking is natural, and small imbalances can be locked in permanently. Once one hand dominated, biology had no way back. The entire genetic and metabolic architecture was built upon that choice.
This is why the Murchison finding is so crucial. Unlike panspermia, it doesn’t shift the biological problem elsewhere. Instead, it provides a physical boundary condition for Earth’s chemistry. The source of that cosmic bias is thought to be physical: asymmetric photolysis, where circularly polarized light from a nearby supernova or neutron star selectively destroyed one “hand” of molecules in the presolar dust cloud.
The cosmos didn’t hand us life. It may have simply handed our planet a “thumb on the scales,” a slight abiotic nudge that Earth’s own chemistry then amplified into the irreversible, homochiral standard for all life that followed.
The Murchison meteorite, then, doesn’t contain a riddle; it contains a clue. Somewhere between starlight and rock chemistry, a slight asymmetry arose. And then, Earth inherited it and amplified it.14
13. LUCA in the Phylogenetic Sky (Without Diamonds)
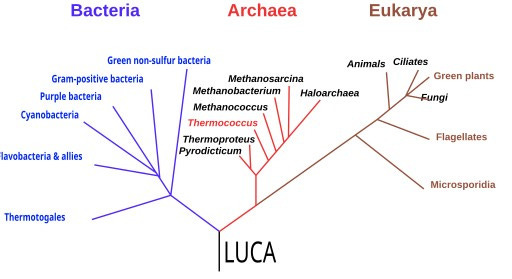
While chemists wrestled with chirality and geologists mapped energy sources, molecular biology was quietly assembling its own time machine. In the 1970s, Carl Woese began comparing ribosomal RNA sequences across organisms. His work overturned the neat two-kingdom model of “prokaryotes” and “eukaryotes.” Instead, Woese revealed a third domain: the Archaea, as distinct from bacteria as either is from us.15
This re-drawing of the tree of life did more than shuffle taxonomy. It offered a new way to probe deep time. If the ribosome’s RNA is universal, then by comparing its sequence across species, scientists could triangulate back to the features of the Last Universal Common Ancestor (LUCA).16
The portrait that emerged surprised many and remains hotly debated.
One prominent hypothesis, championed by Bill Martin and others, paints LUCA as a metabolically sophisticated organism: anaerobic, autotrophic, living on hydrogen and carbon dioxide, and powered by proton gradients across membranes. In this view, LUCA looks suspiciously vent-like.
Other phylogenetic models, however, paint a different picture, suggesting a LUCA that was perhaps a heterotroph living in a cooler, potassium-rich environment, already equipped with a surprisingly complex genetic and replication system.
Whichever way you look at it, though, LUCA was not a simple blob.
It was already a metabolically sophisticated organism. It carried a genetic code, ribosomes, and a rudimentary metabolism. In short, LUCA was not the first step across the threshold from chemistry to biology. It was the last common node of all living things we know today.17
This distinction matters.
LUCA is not “the origin of life.” It is misleading to think of it as one organism; LUCA was a population or community with genetic exchange rather than a single organism. LUCA is the last survivor of an earlier diversity of protocells, the lineage that outcompeted or outlasted all the others. Its existence shows that by the time life was common enough to leave descendants, the core machinery – ribosomes, ATP synthase, genetic code – was already in place.
Origins research, therefore, is not about recreating LUCA. It is about understanding the chemical pathways and thresholds that came before LUCA, in the hazy prehistory where molecules, compartments, energy, and information were still learning how to fit together.
From Darwin’s pond to Woese’s tree, the story arcs not toward certainty but toward constraint. We do not know the exact path, but we know what the destination looked like. LUCA stands as a molecular fossil, proof that by 3.5–4.0 billion years ago, biology had already crossed the decisive boundaries. The question is how.
14. Life by a Thousand Crossings
By now the story arcs from Darwin’s “warm little pond” to Pasteur’s swan-neck flasks, Oparin’s soup, Miller’s sparks, Eigen’s equations, Spiegelman’s Monster, Cech and Altman’s ribozymes, Gilbert’s RNA World, Sutherland’s synthetic chemistry, Joyce’s replicating ribozymes, Szostak’s vesicles, Martin and Lane’s vent engines, the organics of Murchison and 67P, the bias of chirality, and Woese’s LUCA. Each milestone chipped away at the gap between chemistry and biology. None gave the full answer.
But we now know that life emerged not by a lightning bolt moment, nor an alien delivery, nor a single spark or a moment of creation.
Life emerged by crossing a series of thresholds, each narrowing the wide field of chemistry until only biology remained possible.18 But these thresholds were not a linear sequence of steps. They were a messy, intertwined, co-evolutionary process.
The concentration threshold – when minerals, membranes, or coacervates gathered wandering molecules – was essential, but it evolved with the autocatalytic threshold, as containment made self-sustaining reactions possible. The heredity threshold, when copying fidelity rose above the error threshold, was inseparable from the chirality threshold, as a single-handed system is far easier to copy reliably.
These problems were solved in tandem. The energy threshold – tapping natural gradients – likely co-evolved with the integration threshold, as membranes that could hold a gradient were selected for. The coding threshold, linking sequences to functions, was the final lock, fusing metabolism and heredity into a single, co-dependent system: the first true cell.
From that point on, chemistry no longer drifted. It strove, competed, and remembered – it resembled biology. The long prelude of matter had ended. The story of life had begun.
This perspective explains why panspermia and meteorites, though rich in molecules, don’t solve the riddle. The hard part was never the delivery of ingredients. It was crossing the coupled thresholds that turn chemistry into something that persists, remembers, and evolves.
That is where this essay leaves us. Origins-of-life research, after a century and a half, has turned mystery into a map of transitions.
But this map only shows the route; it doesn’t explain the engine. What unites these thresholds? What does it cost, in physical terms, for matter to persist against entropy and begin to store information?
That is the focus of the final part: the energetic price of memory at life’s dawn.
I am grateful to
, , , and for their valuable feedback and discussions during the drafting process.We still teach organic synthesis to undergrads this way because it’s systematic, if not always realistic.
For a distillation of decades of failed and semi-successful attempts to make RNA from scratch, why the “stitch-together” approach failed, and why this was a crisis for the RNA World hypothesis, see Orgel’s 2004 review.
For Sutherland’s era-defining synthetic breakthrough, see Powner et al. (2009).
For a review of how prebiotic chemistry can spontaneously organize into lifelike compartments, see Deamer (2017).
Jack Szostak’s group demonstrated how RNA replication can occur within simple vesicles if magnesium ions are sequestered, resolving a long-standing incompatibility (Adamala & Szostak, 2013).
The physics of phase separation and coacervates is reviewed in Hyman et al. (2014).
For the metabolism-first theory, see the Wikipedia page on the Iron–sulfur world hypothesis, which cites most of the foundational work of Wächtershäuser.
For more details, see Kelley et al. (2005), the classic paper on Lost City, with stunning images and accessible explanations for why it matters.
For a highly readable synthesis of how the universal use of proton gradients in living cells may reflect inheritance from natural geochemical batteries at ancient hydrothermal vents, see Lane & Martin (2012). The article is accessible to anyone with a basic background in college chemistry.
For a concise and readable account of the proton gradient origin hypothesis, which also addresses responses to critiques of convergent evolution, see Lane (2017).
For a summary of the organic findings in the Murchison meteorite and the enantiomeric bias among its amino acids, see the Murchison meteorite Wikipedia entry.
I struggle to think of a more incredible scene ever captured by human technology.
For a readable review of how small chiral biases (from space or chemistry) can be transformed into near-total homochirality through amplification mechanisms like the Soai reaction and Viedma ripening, see Blackmond (2009).
In a classic “if-only” twist that typifies the maddening beauty of scientific puzzles, the only amino acid detected on comet 67P/Churyumov–Gerasimenko was glycine, which is the sole non-chiral amino acid among the twenty standard building blocks of terrestrial life.
For the shift to three domains and ribosomal phylogenetics, see Biology LibreTexts on Carl Woese.
For a readable summary of LUCA’s characteristics, see the LUCA Wikipedia page.
For new findings on LUCA and its evolutionary context, see Popular Mechanics’ reporting on recent LUCA studies.
For a modern review of the many intertwined chemical and evolutionary thresholds that likely mark the transition from chemistry to biology, see Jeancolas, Malaterre, and Nghe (2020). The article is readable and synthesizes current interdisciplinary thinking on how life really gets started.

