Jumping Genes: Barbara McClintock’s Rewriting of Genetics
How a Brilliant Scientist's Rejected Discovery Revolutionized Our Understanding of the Genome
.. and these [fundamental laws and facts of physical science] are now so firmly established that the possibility of their ever being supplanted in consequence of new discoveries is exceedingly remote.”
Albert Michelson, recipient of the 1907 Nobel Prize in Physics [in 1894].
“Anything found to be true of E. coli must also be true of elephants.”
Jacques Monod, recipient of the 1965 Nobel Prize in Physiology or Medicine [in 1954].
The Fallacy of "Finished" Science

A corollary to Arthur C. Clarke’s First Law1 might read: “When a distinguished scientist declares their field complete, they are almost certainly wrong.” Such pronouncements do concede a few “pesky details,” but assume that explanations for those details will nestle comfortably inside existing theory.
Reality often disagrees. Nature, not being obligated to our current theories, replies with baffling observations to our carefully designed experiments, placing the devil squarely back in the details. And the explanations of those details have a habit of upending our existing theoretical frameworks.
For example, within a generation of Albert A. Michelson’s 1894 statement, relativity and quantum theory upended the ‘finished’ physics Michelson invoked. Ironically, this happened thanks in part to his own experiments disproving the existence of the ether.
And within three decades of Jacques Monod’s 1954 quip that what is true of E. coli is true of elephants, Barbara McClintock (1902-1992) had received the 1983 Nobel Prize in Physiology or Medicine for starting an equally radical but quieter upheaval. Monod’s 1961 lac‑operon model of bacterial gene regulation2 convinced many that life’s control circuits were finally understood. Yet those same concepts would later illuminate the roving transposable elements in maize that McClintock had already glimpsed in the 1940s. McClintock’s discovery of “jumping genes” made it clear that, among other things, E. coli and elephants are very different indeed.
Although most of McClintock’s peers had found their proposed existence too radical and were dismissive, these transposable elements (or transposons) would eventually force biologists to rethink what a gene could be. Yet while physics’ revolution is textbook lore, the story of transposons — and how one overlooked but determined researcher changed genetics — remains largely unknown.
The Early Years
Barbara McClintock first discovered transposons, or “jumping genes,” in maize in 1948. Today, we know that these mobile DNA segments are found in all eukaryotes and account for roughly half of the human genome. However, most transposable elements in complex eukaryotes like us have been deactivated due to accumulated mutations over millions of years of evolution, earning them the moniker of “junk DNA.”
But that junk has begun to prove useful. In 1997, nearly half a century after McClintock’s discovery of transposons, Zoltán Ivics and his team at the Paul Ehrlich Institute in Germany “woke up” one such deactivated transposon from its millions of years of evolutionary sleep. They named it the Sleeping Beauty Transposable Element. It remains a harbinger of the tantalizing possibilities in gene therapy and genetic engineering — but we are getting ahead of ourselves.
Understanding how the idea of the ‘gene’ evolved is crucial to appreciating why McClintock’s claims sounded so radical to her contemporaries. The 20th century saw the explosive growth of the field of genetics. At the turn of the century, researchers “rediscovered” the work of the 19th-century friar Gregor Mendel, who had studied the heredity of pea plants in his abbey garden. In 1905, the British biologist William Bateson coined the term “genetics”, followed shortly by the word “gene” which the Danish botanist Wilhelm Johannsen originated in 1909. Initially, scientists used “gene” as a theoretical abstraction to explain Mendelian inheritance. By 1915, however, there was some evidence from fruit fly (Drosophila) studies to propose that a “gene” was a physical reality, situated in the chromosomal structure within a cell.

Drosophila would go on to become the model organism in genetic studies for most of the 20th century. But Barbara McClintock, and her mentors at Cornell University, studied maize. The kernels were “almost diagrammatic expression of genetic traits,” wrote McClintock’s biographer Evelyn Fox Keller in A Feeling for the Organism: The Life and Work of Barbara McClintock. As a PhD student in botany, McClintock improved a staining technique that both increased slide durability and enhanced contrast, allowing her to identify and characterize individual maize chromosomes and map breakages. She established that maize has ten chromosomes and that the exchange of genetic information is accompanied by an exchange of chromosomal material — at last, undeniable empirical evidence of the chromosomal basis of genetics. “Chromosomes, heteromorphic in two regions, have been shown to exchange parts at the same time they exchange genes assigned to these regions,” McClintock reported in a 1931 paper now considered a cornerstone of experimental genetics.
Throughout the 1930s, McClintock studied the relation between cell components and genes, establishing the link between cytology and genetics. During those years, she moved from Cornell to Caltech to the University of Missouri, finally accepting, in 1941, a permanent position in the Department of Genetics at Cold Spring Harbor Laboratory, where she spent the rest of her long and consequential career in relative obscurity. In 1944 — the same year she became the third woman elected to the National Academy of Sciences — McClintock began systematic studies of spotted maize kernels.
The Strange Case of Jumping Genes
Like every scientist, McClintock stood on the shoulders of giants. In the early 1900s, Hugo de Vries established the fact that some color patterns in certain leaves and flowers — a phenomenon called variegation — do not strictly follow Mendelian inheritance. In an apparent violation of Mendelian principles, variegated plants can give rise to non-variegated ones and non-variegated ones to variegated ones3.
Mendelian genetics, treating the gene as an indivisible unit of inheritance, remained prevalent until the 1940s. In 1914, RA Emerson tried to reconcile the variegation in maize with Mendelian inheritance by positing the existence of a temporary inhibitor of pigmentation whose loss restored color. His studies also implied that variegation is a phenotype of an unstable gene4.
By 1938, Marcus Rhoades, McClintock’s old colleague from Cornell, had identified a maize locus that could render a stable gene unstable. He concluded that a gene’s stability depended on its genetic environment and rejected the view that mutable alleles were simply “sick.” His data were telling him that the presence or absence of an unlinked second gene could decide a mutable gene’s stability, and hence an unstable gene could mutate to its normal stable form.
McClintock started studying mutable genes incidentally while researching the breakage-fusion-bridge cycle in chromosome 9 of maize at Cold Spring Harbor. Chromosome 9 contains genes responsible for pigmentation in the maize kernel5.
To reveal novel mutations, McClintock self-pollinated maize plants in which each parent contributed a broken chromosome 9. In 1944 and 1945, she reported mutants that showed variegation from the recessive to the dominant phenotype. The following year, she pinpointed a fragile site on chromosome 9 — Ds for dissociation — that fractured only when a second locus, Ac (activator), was present; together they became the Ac/Ds system. More bizarrely, Ds sometimes jumped to a new chromosomal address, altering the kernel’s color in ways no static‑gene model could explain.6
That McClintock did the labor-intensive and logistically complex work of mapping maize genetics is a testament to her formidable work ethic: a maize geneticist must, first and foremost, grow the crop during the oppressive summer months, and take extreme care in preventing cross-pollination on the field, and catalogue hundreds of samples off the field. That she discovered transposition by linking the resulting phenotypic features and the underlying chromosomal mechanism using, essentially, only a microscope is a testament to her extraordinary scientific intuition and intimate grasp of her experimental system7. Transposable elements, we now know, are short DNA segments that encode transposase proteins capable of moving the element elsewhere in the genome. Yet when McClintock began, geneticists had not even proved that hereditary material was deoxyribonucleic acid (DNA).

By the end of 1950, McClintock was aware of the radical implications of her discoveries - and apprehensive about the prospect of sharing them at the 1951 Cold Spring Harbor Symposium. Her apprehensions proved well-founded, even understated. The talk was met with reactions ranging from stony silence to outspoken opposition, as were her four more attempts (1955, 1956, 1960, 1965). “Ahead of her time” is how the decades of resistance to her ideas is explained in retrospect. McClintock admitted to her biographer Evelyn Fox Keller that the disastrous 1951 symposium really “knocked her” as she was convinced that she was right. However, she absorbed herself in analyzing a new system she designated as Suppressor-mutator (Spm).
A Scientific Community Unprepared
McClintock’s ideas were indeed ahead of their time, yet her ideas were also considered convoluted and obscure by her contemporaries. An ongoing revolution in biological thought was to blame.
The 1950s saw the advent of molecular biology, a movement led by an influx of physicists determined to apply their methodology to the pressing questions of biology. Perhaps the most famous among these now is James Watson, a frequent visitor to Cold Spring Harbor, who with Francis Crick in 1953 proposed the double-helix structure of DNA, using then-unpublished data obtained by their colleague Rosalind Franklin (without attribution and without her permission).

Crick then went on to propose the so-called “central dogma” of molecular biology8: once information flows from DNA to RNA to protein, it cannot flow back. Such one‑way traffic left no room for DNA segments that move, much less regulate other genes. Crick’s central dogma solidified the neoclassical view of the gene in which each gene was thought to be responsible for the synthesis of one single polypeptide. Even as conflicting information amassed, it remained prevalent until the 1970s.
Molecular biologists also imported a mechanistic, Newtonian mindset from Physics that treated the cell as nothing more than a collection of molecules. This presented another hurdle to the acceptance of transposable elements, whose existence McClintock deduced from decidedly “pre-molecular” studies (performed even before the double-helix structure of DNA was elucidated in 1953).
The functions of a living cell were now described in terms of its components, the molecules that make up the cell. Findings in E. coli or phages were assumed universal: if it held for the bacterium, it must hold for the elephant. As research funding, and hence new talent, flowed to these simple systems, field geneticists like McClintock, who studied higher-level organisms like maize, found fewer peers able to grasp their results or even willing to put in the effort.
McClintock’s capacity to work alone, immersing herself in her research to the exclusion of almost everything else, sustained her during the decades it took the scientific community to accept her findings. It is a testament to the robustness of the scientific method that later discoveries in molecular biology ultimately vindicated her; the mountain, at last, came to Mohammed.
While molecular biologists spent three decades “rediscovering” transposition in simpler systems, McClintock kept ploughing ahead with her maize work. During those same years, the National Academy of Sciences enlisted her to train scientists in Central and South America to collect and preserve indigenous maize strains threatened by industrial agriculture. For that effort, she received the Kimber Genetics Award in 1967 and the National Medal of Science in 1970. These, however, were a meager consolation for the rejection of her most monumental work.
Paradigm Shift, or May the Best Explanation Win
In his highly influential book The Structure of Scientific Revolutions, the philosopher Thomas Kuhn distinguishes “normal science,” where progress is cumulative, from “revolutionary science,” where anomalies force a conceptual overhaul. The 1960s‑70s rediscovery of transposition exemplifies the latter pattern: molecular biologists, constrained by their own tools, had to re‑encounter what Barbara McClintock had already seen in maize.
McClintock came to transposable elements while explaining the “exception within exceptions” behavior of spotted maize kernels using the pre-molecular conceptual framework of biology. The generation of molecular biologists after McClintock had to follow their own route to transposition. There was no other way: because molecular biologists (justifiably) trusted their own methods and (unjustifiably) discounted pre‑molecular work, acceptance of McClintock’s conclusions had to await their rediscovery.
That rediscovery unfolded in rapid steps. In 1963, A. L. Taylor showed that Mu bacteriophage inserts randomly into bacterial chromosomes, unlike classic, site‑specific transduction. Late‑1960s work in E. coli uncovered mobile “insertion sequences” native to the genome, and studies of Salmonella typhimurium linked rampant drug‑resistance spread to genes that could jump at will. By 1977, Gerald Fink demonstrated transposition in yeast — so reminiscent of McClintock’s maize Suppressor‑mutator system (Spm) that he borrowed her nomenclature. From there, “jumping genes” turned up in organism after organism. By the mid‑1970s, what once seemed a maize oddity had become a universal phenomenon.
This brings us back to Monod’s lac‑operon model. The model had reframed genes not merely as static blueprints, but as switchable units controlled by adjacent regulatory sequences—a conceptual leap that, applied retrospectively, helped make McClintock’s maize data legible. Her Ac/Ds “controlling elements” had puzzled contemporaries because they altered kernel‑color genes without altering their coding regions; viewed through the operon lens, Ac resembled an inducible activator and Ds a mobile operator whose excision flipped expression from “off” to “on.”
Although the exact molecular logic differed in these two systems9, the conceptual overlap of “genes can be controlled” helped make the case for “controlling elements” in maize. The operon paradigm thus supplied the missing vocabulary—regulatory element, operator, inducer—to interpret Ac/Ds as mobile regulators of gene expression rather than capricious mutations. Once molecular biologists adopted this regulatory mindset in the 1960s–70s, the parallels between bacterial operons and McClintock’s “jumping genes” became unmistakable10.
Kuhn would label this chain of events a paradigm shift; David Deutsch would call it the rise of a better explanation. McClintock’s Ac/Ds model met Deutsch’s two tests for a good explanation as mentioned in Deutsch’s extraordinary book The Beginning of Infinity: Explanations That Transform the World :
Hard‑to‑vary: Kernel color, chromosomal breakpoints, and a movable DNA element were interlocked; change one part and the story collapsed. In contrast, rival notions of temporary inhibitors or “sick” alleles could be patched endlessly.
Wide reach: Once posited, mobile DNA explained maize anomalies and predicted behavior later observed in bacteria, yeast, and humans.
Resistance, then, was largely sociological and not epistemic once molecular evidence had accumulated. When molecular data caught up, the community adopted the hard‑to‑vary, far‑reaching account because no alternative matched its explanatory power. Progress here is both Kuhnian paradigm replacement and Deutschian triumph of the best explanation.
The long overdue recognition of McClintock’s monumental discoveries came as a flood. To think that had molecular biology moved more slowly, McClintock’s most monumental discovery might have gone unrecognized! Yet she never doubted her work’s merit. “I knew I was right,” she told biographer Evelyn Fox Keller, months before becoming, in 1983, the first (and still only) sole female winner of the Nobel Prize in Physiology or Medicine.

From Maize to Medicine
It is now established that all eukaryotic genomes are peppered by transposable elements in considerable numbers. They fall into two broad classes: Class I “copy‑and‑paste” retrotransposons and Class II “cut‑and‑paste” DNA transposons. The transposons’ profligacy is because of their tendency to accumulate by copying themselves to new locations in the genome.
The identifying feature of the class II transposable elements, which include McClintock’s maize transposons and the Sleeping Beauty transposable elements, is the presence of tandem inverted repeat (TIR) sequences. The transposase protein that is encoded by the DNA segment flanked by these TIRs binds to the specific recognition sequences in these TIRs, excises the entire cassette, and then inserts it into another site in the host genome. The vast majority of class II transposable elements, however, are actually “fossils”: they have lost their ability to move within the genome because of accumulated mutations in the part that codes for transposase proteins. These “fossils” still contain the TIRs, though. And introducing transposase proteins can make them mobile again.
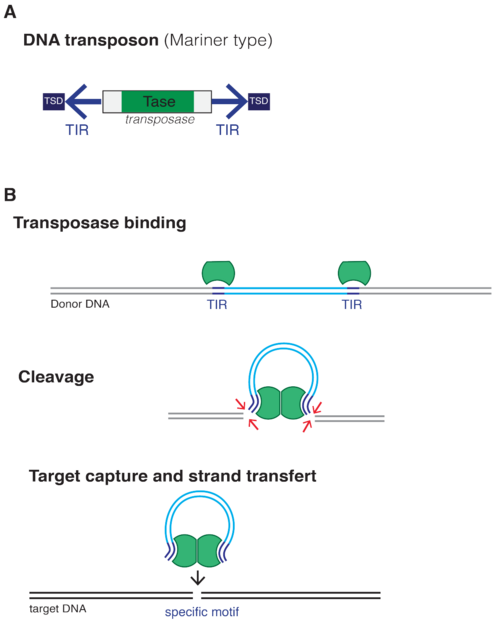
This modularity is what makes Sleeping Beauty (and other transposon systems like PiggyBac) so promising for gene therapy and genetic engineering applications. Insert any therapeutic payload between its TIRs, give the cell an SB transposase, and the cassette integrates with viral‑level efficiency yet without viral proteins, avoiding waking up the body’s immune response. Since its reactivation by Ivics and his team in 1997, SB has advanced through more than two dozen pre‑clinical studies and a dozen phase I/II trials— making it the most clinically advanced DNA transposon.
Why, then, do viral vectors still dominate? Ivics and Amberger argue that the imbalance is historical: viral tools matured a decade before Sleeping Beauty was “woken,” establishing manufacturing infrastructure and regulatory familiarity. The main advantages of transposon-based vectors are their low costs and high manufacturing speeds, two important considerations when it comes to personalized medicine. Amberger and Ivics take a long view where the goal is not to compete with viral vectors, but “to venture into novel areas where [viral vectors] are not an option.”
McClintock once said she had “a feeling for the organism”11, an intuitive rapport with the maize plant as a whole. Sleeping Beauty extends that feeling to therapy: a century after her first spotted maize, the same cut‑and‑paste logic may let clinicians patch broken genes in people. The jump from cornfield to clinic is long, but the principle—mobile DNA rewriting its own story—remains unmistakably hers.
“When a distinguished but elderly scientist states that something is possible, he is almost certainly right. When he states that something is impossible, he is very probably wrong.”
In 1961 Jacques Monod and François Jacob described the lac operon of E. coli: three contiguous genes for lactose metabolism (lacZYA) are transcribed from a single promoter but kept “off” by a repressor bound to an adjacent operator; lactose (via allolactose) inactivates the repressor, switching the operon “on.” This inducible, switch‑like control became the canonical model for gene regulation.
If variegation in maize, i.e., the spots in maize kernels, were a genetic trait that followed Mendelian principles, then this trait would have alternate forms (“spotted” and “non-spotted”). These forms are called alleles, and one of the alleles is dominant over the other (“spotted” over “non-spotted” or vice versa). The alleles of a particular gene are arranged in their specific fixed positions, or loci, in the chromosome like beads on a string. During reproduction, gametes are formed by random segregation such that each gamete carries only one allele for each gene. This step is called meiosis. These gametes fuse during fertilization, bringing both alleles of a gene together again. The physical appearance, or phenotype, of a trait in the offspring reflects the dominant allele. If we consider “spotted” as dominant, then a spotted kernel would indicate that at least one of the parents had spotted kernels and non-spotted would mean that both parents had non-spotted kernels.
Emerson noted that the size of the pigmented tissue was directly correlated with the probability of inheritance of the wild-type allele of the pigmentation gene.
Two of the genetic markers McClintock focused on were C1 (dominant inhibitor I, recessive c) and Bz(bronze, recessive bz). C1 is required for seed color. In the presence of the I allele, which is a dominant inhibitor of C, the kernels are colorless. Bz is required to make the kernel purple rather than bronze, so the recessive bz in the absence of the dominant Bz will give a bronze kernel.
Chromosome 9 carries a color activator (C/c) and a hue modifier (Bz/bz); the inhibitor I silences C.
· I Bz Ds → Ds excises the entire I Bz block. With I and dominant Bz gone, recessive bz pairs with C, producing bronze sectors.
· I Ds Bz → After Ds jumps between I and Bz, it now removes only I. C is freed while Bz remains, yielding purple sectors.
(In shorthand: C bz │ I Bz Ds → bronze; C bz │ I Ds Bz → purple.)
First published in 1958, Crick stated that the flow of biological information was strictly unidirectional: “Once ‘information’ has passed into protein it cannot get out again.”
Ac functioned in trans (via its protein product), whereas a classic operator like lac acts in cis, but both reveal that DNA segments can regulate other loci. In the maize system, Ac provided the activating ‘signal’ (much like an inducible regulator), while Ds supplied the regulatory ‘switch’ that could literally change position.
By the early 1970s, once cloned insertion sequences in bacteria displayed terminal repeats and target‑site duplications familiar to maize cytologists, the conceptual gulf all but disappeared.
“It might seem ridiculous, but I have a feeling for the organism”, quoted in her biography by Evelyn Fox Keller.



